Structured Benefit-Risk Assessment of a New Quadrivalent Meningococcal Conjugate Vaccine (MenACYW-TT) in Individuals Ages 12 Months and Older
Par un écrivain mystérieux
Description
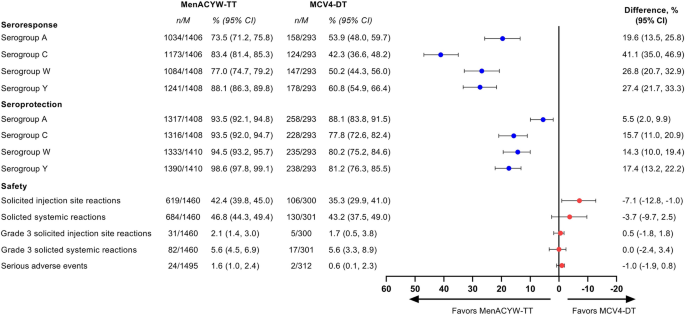
Structured Benefit-Risk Assessment of a New Quadrivalent Meningococcal Conjugate Vaccine (MenACYW-TT) in Individuals Ages 12 Months and Older

Immunogenicity and safety of meningococcal group A, C, W and Y tetanus toxoid conjugate vaccine: review of clinical and real-world evidence

Full article: Immunogenicity and safety of MenACWY-TT, a quadrivalent meningococcal tetanus toxoid conjugate vaccine recently licensed in the United States for individuals ≥2 years of age

Full article: Real-world impact and effectiveness of MenACWY-TT

Meningococcal Serogroup ACWYX Conjugate Vaccine in Malian Toddlers

Meningococcal Serogroup ACWYX Conjugate Vaccine in Malian Toddlers
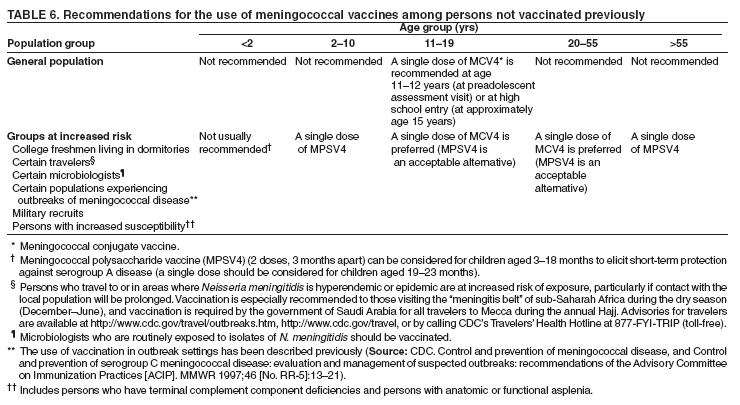
Prevention and Control of Meningococcal Disease
Recommendations of the Advisory Committee on Immunization Practices (ACIP)

ECTRIMS/EAN consensus on vaccination in people with multiple sclerosis: Improving immunization strategies in the era of highly active immunotherapeutic drugs - Susana Otero-Romero, Christine Lebrun-Frénay, Saúl Reyes, Maria Pia Amato, Magda Campins
Full article: Immunogenicity and safety of a booster dose of a quadrivalent meningococcal tetanus toxoid-conjugate vaccine (MenACYW-TT) in adolescents and adults: a Phase III randomized study

An evaluation of emerging vaccines for childhood meningococcal disease – topic of research paper in Economics and business. Download scholarly article PDF and read for free on CyberLeninka open science hub.
depuis
par adulte (le prix varie selon la taille du groupe)







